Sistemas poliméricos mucoadhesivos para la liberación vaginal de fármacos: revisión sistemática
DOI:
https://doi.org/10.17488/RMIB.44.2.4Palabras clave:
Sistemas poliméricos mucoadhesivos, liberación vaginal de fármacos, tratamientoResumen
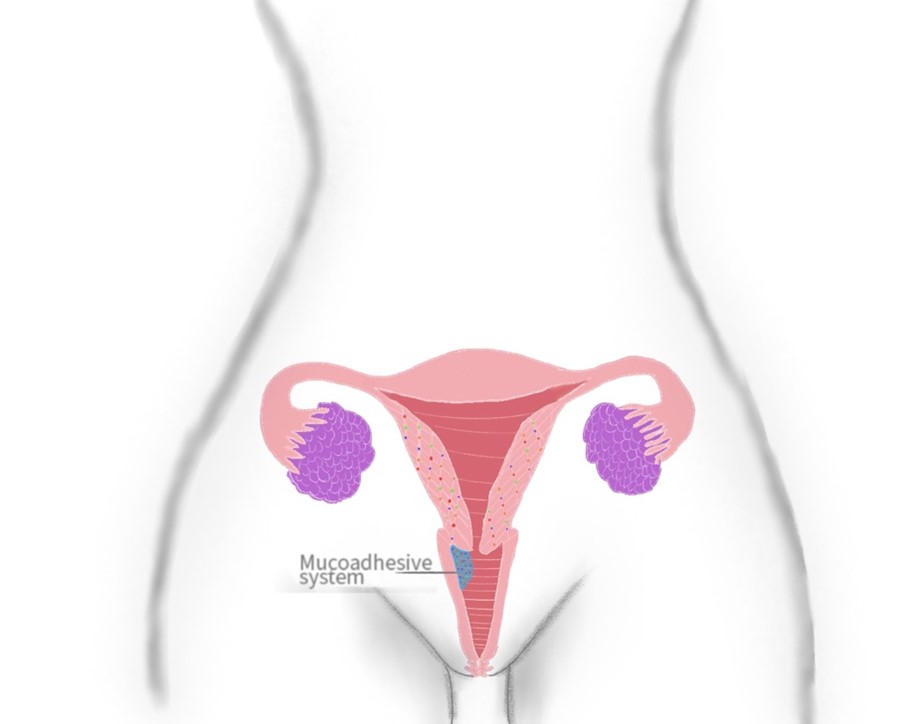
La administración de fármacos por vía intravaginal cuenta con múltiples ventajas a comparación de otras rutas, puede lograr un efecto tanto local como sistémico, las dosis requeridas son menores, facilidad de administración entre otras, hacen que esta forma de administración sea confiable y cómoda. Esta vía de administración puede ser empleada para prevenir y tratar diferentes trastornos, como enfermedades de transmisión sexual, desordenes hormonales, anticonceptivos y tratamiento contra el cáncer. La presentación de las dosis puede variar, desde óvulos, tabletas, anillos, geles, cremas, películas entre otros, agregando a esta, en los últimos tiempos, la característica de mucoadhesividad para reducir el desecho de moléculas activas. Este trabajo se enfoca en las aplicaciones que han tenido los sistemas poliméricos mucoadhesivos en la vía intravaginal. La bibliografía recolectada se obtuvo de bases de datos como Science direct, SciELO, y PubMed Central. Los resultados obtenidos demuestran que la administración de fármacos por vía intravaginal puede ser una forma alternativa para medicación en mujeres, con resultados más estables y prolongados que otras rutas, requiriendo menores dosis y evitando el efecto de primer paso.
Descargas
Citas
D. I. Macht, “On the absorption of drugs and poisons through the vagina,” J. Pharmacol. Exp. Ther., vol. 10, no. 7, pp. 509–522, Jan. 1918. [Online]. Available: https://jpet.aspetjournals.org/content/10/7/509
G. D. Robinson, “Absorption from the human vagina,” BJOG, vol. 32, no. 3, pp. 496–504, Sep. 1927, doi: https://doi.org/10.1111/j.1471-0528.1925.tb06358.x
J. M. Stevens, “Gynaecology from ancient Egypt: The papyrus kahun: A translation of the oldest treatise on gynaocology bas survived from tha ancient world”, Med. J. Aust., vol. 2, no. 25-26, pp. 949–952, Dec. 1975, doi: https://doi.org/10.5694/j.1326-5377.1975.tb106465.x
J. das Neves, F. Notario-Pérez, B. Sarmento, “Women-specific routes of administration for drugs: A critical overview,” Adv. Drug. Deliv. Rev., vol. 176, art. no. 113865, Sep. 2021, doi: https://doi.org/10.1016/j.addr.2021.113865
N. Dobaria, R. Mashru, N. H. Vadia, “Vaginal drug delivery systems: A Review of Current Status,” East Cent. Afr. J. Pharm. Sci., vol. 10, no. 1, pp. 3–13, 2007, doi: https://doi.org/10.4314/ecajps.v10i1.9754
N. J. Alexander, E. Baker, M. Kaptein, U. Karck, L. Miller, E. Zampaglione, “Why consider vaginal drug administration?,” Fertil Steril, vol. 82, no. 1, pp. 1–12, Jul. 2004, doi: https://doi.org/10.1016/j.fertnstert.2004.01.025
A. Hussain, F. Ahsan, “The vagina as a route for systemic drug delivery,” J. Control. Release, vol. 103, no. 2, pp. 301–313, Mar. 2005, doi: https://doi.org/10.1016/j.jconrel.2004.11.034
A. S. M. Abu El- Enin, A. M. Elbakry, R. El Hosary, M. A. Fouad Lotfy, and R. Yahia, “Formulation, development, in vivo pharmacokinetics and pharmacological efficacy evaluation of novel vaginal bioadhesive sustained core-in-cup salbutamol sulphate tablets for preterm labor,” J. Drug Deliv. Sci. Technol., vol. 60, Art. no. 102076, Dec. 2020, doi: https://doi.org/10.1016/j.jddst.2020.102076
J. W. Yoo, K. Dharmala, C. H. Lee, “The physicodynamic properties of mucoadhesive polymeric films developed as female controlled drug delivery system,” Int. J. Pharm., vol. 309, no. 1–2, pp. 139–145, Feb. 2006, doi: https://doi.org/10.1016/j.ijpharm.2005.11.020
A. Jalil, M. H. Asim, N. N. Le, F. Laffleur, B. Matuszczak, M. Tribus, A. Bernkop-Schnürch, “S-protected gellan gum: Decisive approach towards mucoadhesive antimicrobial vaginal films,” Int. J. Biol. Macromol., vol. 130, pp. 148–157, Feb. 2019, doi: https://doi.org/10.1016/j.ijbiomac.2019.02.092
M. T. Sánchez, M. A. Ruiz, H. Castán, M. E. Morales, “A novel double-layer mucoadhesive tablet containing probiotic strain for vaginal administration: Design, development and technological evaluation,” Eur. J. Pharm. Sci., vol. 112, pp. 63–70, Jan. 2018, doi: https://doi.org/10.1016/j.ejps.2017.11.006
A. Martín-Illana, R. Cazorla-Luna, F. Notario-Pérez, L. M. Bedoya, J. Rubio, A. Tamayo, R. Ruiz-Caro, M. D. Veiga, “Smart vaginal bilayer films of Tenofovir based on Eudragit® L100/natural polymer for the prevention of the sexual transmission of HIV,” Int. J. Pharm., vol. 602, Jun. 2021, doi: https://doi.org/10.1016/j.ijpharm.2021.120665
L. Kumar, S. Verma, B. Vaidya, V. Gupta, “Bioadhesive Polymers for Targeted Drug Delivery,” in Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes, V. Mishra, P. Kesharwani, M. C. I. Mohd Amin, A. Iyer, Ed., London, United Kingdom: Academic Press, 2017, ch. 10, pp. 322–362. [Online]. Available: https://doi.org/10.1016/B978-0-12-809717-5.00012-9
S. El Moussaoui, F. Fernández-Campos, C. Alonso, D. Limón, L. Halbaut, M. L. Garduño-Ramirez, A. C. Calpena, M. Mallandrich, “Topical mucoadhesive alginate-based hydrogel loading ketorolac for pain management after pharmacotherapy, ablation, or surgical removal in condyloma acuminata,” Gels, vol. 7, no. 1, art. no. 8, Jan. 2021, doi: https://doi.org/10.3390/gels7010008
C. Karavasili, G. K. Eleftheriadis, C. Gioumouxouzis, E. G. Andriotis, D. G. Fatouros, “Mucosal drug delivery and 3D printing technologies: A focus on special patient populations,” Adv. Drug Deliv. Rev., vol. 176, art. no. 113858, Sep. 2021. doi: https://doi.org/10.1016/j.addr.2021.113858
B. Valamla, P. Thakor, R. Phuse, M. Dalvi, P. Kharat, A. Kumar, D. Panwar, S. B. Singh, P. Giorgia, N. K. Mehra, “Engineering drug delivery systems to overcome the vaginal mucosal barrier: Current understanding and research agenda of mucoadhesive formulations of vaginal delivery,” J. Drug Deliv. Sci. Technol., vol. 70, art. no. 103162, Apr. 2022, doi: https://doi.org/10.1016/j.jddst.2022.103162
P. Tunpanich, E. Limpongsa, T. Pongjanyakul, B. Sripanidkulchai, N. Jaipakdee, “Mucoadhesive sustained-release tablets for vaginal delivery of Curcuma comosa extracts: Preparation and characterization,” J. Drug. Deliv. Sci. Technol., vol. 51, pp. 559-568, Jun. 2019, doi: https://doi.org/10.1016/j.jddst.2019.03.030
S. A. Ballagh, “Vaginal ring hormone delivery systems in contraception and menopause,” Clin. Obstet. Gynecol., vol. 44, no. 1, pp. 106–113, Mar. 2001, doi: https://doi.org/10.1097/00003081-200103000-00014
L. Ci, Z. Huang, Y. Liu, Z. Liu, G. Wei, W. Lu, “Amino-functionalized poloxamer 407 with both mucoadhesive and thermosensitive properties: preparation, characterization and application in a vaginal drug delivery system,” Acta. Pharm. Sin. B., vol. 7, no. 5, pp. 593–602, Sep. 2017, doi: https://doi.org/10.1016/j.apsb.2017.03.002
I. Löwy, “‘Sexual chemistry’ before the Pill: Science, industry and chemical contraceptives, 1920-1960,” Br. J. Hist. Sci., vol. 44, no. 2, pp. 245–274, Jun. 2011, doi: https://doi.org/10.1017/s0007087410000762
R. Palmeira-de-Oliveira, A. S. Oliveira, J. Rolo, M. Tomás, A. Palmeira-de-Oliveira, S. Simões, J. Martínez-de-Oliveira, “Women's preferences and acceptance for different drug delivery routes and products,” Adv. Drug. Deliv. Rev., vol. 182, art. no. 114133, Mar. 2022, doi: https://doi.org/10.1016/j.addr.2022.114133
F. L. Meyskens, V. Graham, M. Chvapil, R. T. Dorr, D. S. Alberts, E. A. Surwit, “A phase I trial of β-all-trans-retinoic acid delivered via a collagen sponge and a cervical cap for mild or moderate intraepithelial cervical neoplasia,” J. Natl. Cancer Inst., vol. 71, no. 5, pp. 921–925, Nov. 1983, doi: https://doi.org/10.1093/jnci/71.5.921
S. F. Lai, M. T. Lam, H. W. R. Li, E. H. Y. Nga, “A randomized double-blinded non-inferiority trial comparing fentanyl and midazolam with pethidine and diazepam for pain relief during oocyte retrieval,” Reprod. Biomed. Online, vol. 40, no. 5, pp. 653–660, May. 2020, doi: https://doi.org/10.1016/j.rbmo.2020.01.021
V. Graham, E. S. Surwit, S. Weiner, F. L. Meyskens, “Phase II trial of β-all-trans-retinoic acid for cervical intraepithelial neoplasia delivered via a collagen sponge and cervical cap,” West. J. Med., vol. 145, no. 2, pp. 192–195, Aug.1986. [Online]. Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1306873/
T. Issat, J. Beta, M. A. Nowicka, T. Maciejewski, A. J. Jakimiuk, “A Randomized, Single Blind, Placebo-Controlled Trial for the Pain Reduction During the Outpatient Hysteroscopy After Ketoprofen or Intravaginal Misoprostol,” J. Minim. Invasive Gynecol., vol. 21, no. 5, pp. 921–927, 2014, doi: https://doi.org/10.1016/j.jmig.2014.04.006
H. El-Refaey, A. Templeton, “Early induction of abortion by a combination of oral mifepristone and misoprostol administered by the vaginal route,” Contraception, vol. 49, no. 2, pp. 111–114, Feb. 1994, doi: https://doi.org/10.1016/0010-7824(94)90085-X
P. Vercellini, G. Barbara, E. Somigliana, S. Bianchi, A. Abbiati, L. Fedele, “Comparison of contraceptive ring and patch for the treatment of symptomatic endometriosis,” Fertil Steril., vol. 93, no. 7, pp. 2150–2161, May. 2010, doi: https://doi.org/10.1016/j.fertnstert.2009.01.071
L. Perioli, V. Ambrogi, C. Pagano, E. Massetti, C. Rossi, “New solid mucoadhesive systems for benzydamine vaginal administration,” Colloids Surf. B, vol. 84, no. 2, pp. 413–420, Jun. 2011, doi: https://doi.org/10.1016/j.colsurfb.2011.01.035
R. Sanz, B. Clares, M. Mallandrich, J. Suñer-Carbó, M. J. Montes, A. C. Calpena, “Development of a mucoadhesive delivery system for control release of doxepin with application in vaginal pain relief associated with gynecological surgery,” Int. J. Pharm., vol. 535, no. 1-2, pp. 393-401, Jan. 2018, doi: https://doi.org/10.1016/j.ijpharm.2017.11.027
V. K. P. de Queiroz, A. M. da Nóbrega Marinho, G. A. M. de Barros, “Analgesic effects of a 5% lidocaine patch after cesarean section: A randomized placebo-controlled double-blind clinical trial,” J. Clin. Anesth., vol. 73, art. no. 110328, Oct. 2021, doi: https://doi.org/10.1016/j.jclinane.2021.110328
Organización Mundial de la Salud (OMS), “Cáncer cervicouterino,” WHO. https://www.who.int/es/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer (accessed 2022).
Drugbank Online, “Ketorolac,” Drugbank Online. https://go.drugbank.com/drugs/DB00465 (accessed Apr. 11, 2021).
A. M. Larish, R. R. Dickson, R. A. Kudgus, R. M. McGovern, J. M. Reid, W. M. Hooten, W. T. Nicholson, L. E. Vaughan, T. L. Burnett, S. K. Laughlin-Tommaso, S. S. Faubion, I. C. Green, “Vaginal Diazepam for Nonrelaxing Pelvic Floor Dysfunction: The Pharmacokinetic Profile,” J. Sex. Med., vol. 16, no. 6, pp. 763–766, Jun. 2019, doi: https://doi.org/10.1016/j.jsxm.2019.03.003
World Health Organization (WHO), “Sexually transmitted infections (STIs),” WHO. https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis) (accessed 2022).
N. L. Calvo, G. Tejada, L. A. Svetaz, A. D. Quiroga, V. A. Alvarez, M. C. Lamas, D. Leonardi, “Development and optimization of a new tioconazole vaginal mucoadhesive film using an experimental design strategy. Physicochemical and biological characterization,” J. Pharm. Biomed. Anal., vol. 205, art. no. 114303, Oct. 2021, doi: https://doi.org/10.1016/j.jpba.2021.114303
R. Mishra, P. Joshi, T. Mehta, “Formulation, development and characterization of mucoadhesive film for treatment of vaginal candidiasis,” Int. J. Pharm. Investig., vol. 6, no. 1, p. 47-55, 2016. [Online]. Available: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4787062/
Z. A. Şenyiǧit, S. Y. Karavana, B. Eraç, Ö. Gürsel, M. H. Limoncu, E. Baloǧlu, “Evaluation of chitosan based vaginal bioadhesive gel formulations for antifungal drugs,” Acta Pharm., vol. 64, no. 2, pp. 139–156, Jun. 2014, doi: https://doi.org/10.2478/acph-2014-0013
P. Bassi, G. Kaur, “Bioadhesive vaginal drug delivery of nystatin using a derivatized polymer: Development and characterization,” Eur. J. Pharm. Biopharm., vol. 96, pp. 173–184, Oct. 2015, doi: https://doi.org/10.1016/j.ejpb.2015.07.018
World Health Organization (WHO), “HIV/AIDS,” WHO. https://www.who.int/news-room/fact-sheets/detail/hiv-aids (accessed Oct. 20, 2021).
C. K. Enggi, H. T. Isa, S. Sulistiawati, K. A. R. Ardika, S. Wijaya, R. M. Asri, S. A. Mardikasari, R. F. Donnelly, A. D. Permana, “Development of thermosensitive and mucoadhesive gels of cabotegravir for enhanced permeation and retention profiles in vaginal tissue: A proof of concept study,” Int. J. Pharm., vol. 609, art. no. 121182, Nov. 2021, doi: https://doi.org/10.1016/j.ijpharm.2021.121182
R. Cazorla-Luna, F. Notario-Pérez, A. Martín-Illana, L. M. Bedoya, A. Tamayo, J. Rubio, R. Ruiz-Caro, M. D. Veiga, “Development and in Vitro/ Ex Vivo Characterization of Vaginal Mucoadhesive Bilayer Films Based on Ethylcellulose and Biopolymers for Vaginal Sustained Release of Tenofovir,” Biomacromolecules, vol. 21, no. 6, pp. 2309–2319, Apr. 2020, doi: https://doi.org/10.1021/acs.biomac.0c00249
N. Nematpour, P. Moradipour, M. M. Zangeneh, E. Arkan, M. Abdoli, L. Behbood, “The application of nanomaterial science in the formulation a novel antibiotic: Assessment of the antifungal properties of mucoadhesive clotrimazole loaded nanofiber versus vaginal films,” Mater. Sci. Eng. C Mater. Biol. Appl., vol. 110, art. no. 110635, May 2020, doi: https://doi.org/10.1016/j.msec.2020.110635
L. S. Dolci, A. Liguori, S. Panzavolta, A. Miserocchi, N. Passerini, M. Gherardi, V. Colombo, A. Bigi, B. Albertini, “Non-equilibrium atmospheric pressure plasma as innovative method to crosslink and enhance mucoadhesion of econazole-loaded gelatin films for buccal drug delivery,” Colloids Surf. B, vol. 163, pp. 73–82, Mar. 2018, doi: https://doi.org/10.1016/j.colsurfb.2017.12.030
L. S. Dolci, B. Albertini, M. F. di Filippo, F. Bonvicini, N. Passerini, S. Panzavolta, “Development and in vitro evaluation of mucoadhesive gelatin films for the vaginal delivery of econazole,” Int. J. Pharm., vol. 591, art. no. 119979, Dec. 2020, doi: https://doi.org/10.1016/j.ijpharm.2020.119979
N. Lupo, B. Fodor, I. Muhammad, M. Yaqoob, B. Matuszczak, A. Bernkop-Schnürch, “Entirely S-protected chitosan: A promising mucoadhesive excipient for metronidazole vaginal tablets,” Acta Biomater., vol. 64, pp. 106–115, Dec. 2017, doi: https://doi.org/10.1016/j.actbio.2017.10.014
V. H. S. Araujo, M. P. C. de Souza, G. C. Carvalho, J. L. Duarte, M. Chorilli, “Chitosan-based systems aimed at local application for vaginal infections,” Carbohydr. Polym., vol. 261, art. no 117919, Mar. 2021, doi: https://doi.org/10.1016/j.carbpol.2021.117919
M. Campaña-Seoane, A. Pérez-Gago, G. Vázquez, N. Conde, P. González, A. Martinez, X. Martínez, L. García Varela, M. Herranz, P. Aguiar, A. Fernández-Ferreiro, R. Laguna, F. J. Otero-Espinar, “Vaginal residence and pharmacokinetic preclinical study of topical vaginal mucoadhesive W/S emulsions containing ciprofloxacin,” Int. J. Pharm., vol. 554, pp. 276–283, Jan. 2019, doi: https://doi.org/10.1016/j.ijpharm.2018.11.022
World Health Organization (WHO), “Herpes simplex virus,” WHO. https://www.who.int/news-room/fact-sheets/detail/herpes-simplex-virus (accessed 2022).
M. Ijaz, J. A. Griessinger, A. Mahmood, F. Laffleur, A. Bernkop-Schnürch, “Thiolated Cyclodextrin: Development of a Mucoadhesive Vaginal Delivery System for Acyclovir,” J. Pharm. Sci., vol. 105, no. 5, pp. 1714–1720, May 2016, doi: https://doi.org/10.1016/j.xphs.2016.03.009
E.-M. Pacheco-Quito, L.-M. Bedoya, J. Rubio, A. Tamayo, R. Ruiz-Caro, M.-D. Veiga, “Layer-by-layer vaginal films for acyclovir controlled release to prevent genital herpes,” Int. J. Pharm., vol. 627, art. no. 122239, Nov. 2022, doi: https://doi.org/10.1016/j.ijpharm.2022.122239
D. Ramyadevi, K. S. Rajan, B. N. Vedhahari, K. Ruckmani, N. Subramanian, “Heterogeneous polymer composite nanoparticles loaded in situ gel for controlled release intra-vaginal therapy of genital herpes,” Colloids Surf. B, vol. 146, pp. 260–270, Oct. 2016, doi: https://doi.org/10.1016/j.colsurfb.2016.06.022
Centers for Disease Control and Prevention (CDC), “Sexually Transmitted Diseases (STDs), Prevention,” Sexually Transmitted Diseases (STDs), Prevention. https://www.cdc.gov/std/prevention/default.htm (accessed 2022).
S. Gupta, R. Gabrani, J. Ali, S. Dang, “Exploring Novel Approaches to Vaginal Drug Delivery,” Recent Pat. Drug. Deliv. Formul., vol. 5, no. 2, pp. 82–94, May 2011, doi: https://doi.org/10.2174/187221111795471418
Centers for Disease Control and Prevention (CDC), “HIV,” HIV Basics. https://www.cdc.gov/hiv/basics/whatishiv.html (accessed 2022).
F. Notario-Pérez, R. Cazorla-Luna, A. Martín-Illana, R. Ruiz-Caro, A. Tamayo, J. Rubio, M. D. Veiga, “Optimization of tenofovir release from mucoadhesive vaginal tablets by polymer combination to prevent sexual transmission of HIV,” Carbohydr. Polym., vol. 179, pp. 305–316, Jan. 2018, doi: https://doi.org/10.1016/j.carbpol.2017.10.001
F. Notario-Pérez, A. Martín-Illana, R. Cazorla-Luna, R. Ruiz-Caro, L. M. Bedoya, J. Peña, M. D. Veiga, “Development of mucoadhesive vaginal films based on HPMC and zein as novel formulations to prevent sexual transmission of HIV,” Int. J. Pharm., vol. 570, art. no. 118643, Oct. 2019, doi: https://doi.org/10.1016/j.ijpharm.2019.118643
C. K. Sahoo, P. Kumar Nayak, D. K. Sarangi, T. K. Sahoo, “Intra Vaginal Drug Delivery System: An Overview,” Am. J. Adv. Drug. Deliv., vol. 1, no. 1, pp. 43–55, Apr. 2013, [Online]. Available: https://www.primescholars.com/articles/intra-vaginal-drug-delivery-systeman-overview.pdf
A. Hussain, F. Ahsan, “The vagina as a route for systemic drug delivery,” J. Control Release, vol. 103, no. 2, pp. 301–313, Mar. 2005, doi: https://doi.org/10.1016/j.jconrel.2004.11.034
World Health Organization (WHO), “Endometriosis,” WHO. https://www.who.int/news-room/fact-sheets/detail/endometriosis (accessed Oct. 20, 2021).
K. Carlström, H. Pschera, N. O. Lunell, “Serum levels of oestrogens, progesterone, follicle-stimulating hormone and sex-hormone-binding globulin during simultaneous vaginal administration of 17β-oestradiol and progesterone in the pre- and post-menopause,” Maturitas, vol. 10, no. 4, pp. 307–316, Dec. 1988, doi: https://doi.org/10.1016/0378-5122(88)90066-7
J. Su, K. Sripanidkulchai, J. M. Wyss, B. Sripanidkulchai, “Curcuma comosa improves learning and memory function on ovariectomized rats in a long-term Morris water maze test,” J. Ethnopharmacol., vol. 130, no. 1, pp. 70–75, Jul. 2010, doi: https://doi.org/10.1016/j.jep.2010.04.012
Centers for Disease Control and Prevention (CDC), “Gynecologic Cancers,” Gynecologic Cancers. https://www.cdc.gov/cancer/gynecologic/index.htm (accessed 2022).
M. Crespo, “Cyclometallated platinum(IV) compounds as promising antitumour agents,” J. Organomet. Chem., vol. 879, pp. 15–26, Jan. 2019, doi: https://doi.org/10.1016/j.jorganchem.2018.10.008
Organización Panamericana de la Salud (OPS), “Cáncer cervicouterino,” OPS. https://www.paho.org/es/temas/cancer-cervicouterino (accessed 2022).
S. Mukherjee, “The Emperor of all Maladies”. New York: Scribner Book Company, 2010, pp. 592.
S. Zong, X. Wang, Y. Yang, W. Wu, H. Li, Y. Ma, W. Lin, T. Sun, Y. Huang, Z. Xie, Y. Yue, S. Liu, X. Jing, “The use of cisplatin-loaded mucoadhesive nanofibers for local chemotherapy of cervical cancers in mice,” Eur. J. Pharm. Biopharm., vol. 93, pp. 127–135, Apr. 2015, doi: https://doi.org/10.1016/j.ejpb.2015.03.029
S. Ahmad, “Kinetic aspects of platinum anticancer agents,” Polyhedron, vol. 138, pp. 109–124, Dec. 2017, doi: https://doi.org/10.1016/j.poly.2017.09.016
M. R. Vakili, W. Mohammed-Saeid, A. Aljasser, J. Hopwood-Raja, B. Ahvazi, Y. Hrynets, M. Betti, A. Lavasanifar, “Development of mucoadhesive hydrogels based on polyacrylic acid grafted cellulose nanocrystals for local cisplatin delivery,” Carbohydr. Polym., vol. 255, art. no. 117332, Mar. 2021, doi: https://doi.org/10.1016/j.carbpol.2020.117332
U. Aggarwal, A. K. Goyal, G. Rath, “Development and characterization of the cisplatin loaded nanofibers for the treatment of cervical cancer,” Mater. Sci. Eng. C Mater. Biol. Appl., vol. 75, pp. 125–132, Jun. 2017, doi: https://doi.org/10.1016/j.msec.2017.02.013
D. A. Woolfson, D. F. McCafferty, P. A. McCarron, J. H. Price, “Liquid Scintillation Spectrometry of 5-Fluorouracil in Cervical Tissue Folloing in Vitro Surface Application of a Bioadhesive Cervical Patch,” Pharm. Res., vol. 11, no. 9, pp. 1315–1319, Sep. 1994, doi: https://doi.org/10.1023/a:1018950613353
A. D. Woolfson, D. F. McCafferty, P. A. McCarron, J. H. Price, “A bioadhesive patch cervical drug delivery system for the administration of 5-fluorouracil to cervical tissue,” J. Control. Release, vol. 35, no. 1, pp. 49–58, Jul. 1995, doi: https://doi.org/10.1016/0168-3659(95)00018-4
M. A. Hollingsworth, B. J. Swanson, “Mucins in cancer: Protection and control of the cell surface,” Nat. Rev. Cancer, vol. 4, no. 1, pp. 45–60, Jan. 2004, doi: https://doi.org/10.1038/nrc1251
C. Li, Z. Liu, X. Yan, W. Lu, Y. Liu, “Mucin-controlled drug release from mucoadhesive phenylboronic acid-rich nanoparticles,” Int. J. Pharm., vol. 479, no. 1, pp. 261–264, Feb. 2015, doi: https://doi.org/10.1016/j.ijpharm.2014.12.011
L. Ci, Z. Huang, Y. Liu, Z. Liu, G. Wei, W. Lu, “Amino-functionalized poloxamer 407 with both mucoadhesive and thermosensitive properties: preparation, characterization and application in a vaginal drug delivery system,” Acta Pharm. Sin. B, vol. 7, no. 5, pp. 593–602, Sep. 2017, doi: https://doi.org/10.1016/j.apsb.2017.03.002
F. D. Victorelli, G. M. F. Calixto, K. C. dos Santos, H. H. Buzzá, M. Chorilli, “Curcumin-loaded Polyethyleneimine and chitosan polymer-based Mucoadhesive liquid crystalline systems as a potential platform in the treatment of cervical Cancer,” J. Mol. Liq., vol. 325, art. no. 115080, Mar. 2021, doi: https://doi.org/10.1016/j.molliq.2020.115080
K. Berginc, S. Suljaković, N. Škalko-Basnet, A. Kristl, “Mucoadhesive liposomes as new formulation for vaginal delivery of curcumin,” Eur. J. Pharm. Biopharm., vol. 87, no. 1, pp. 40–46, Feb. 2014, doi: https://doi.org/10.1016/j.ejpb.2014.02.006
Descargas
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2023 Revista mexicana de ingeniería biomédica.

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial 4.0.
Una vez que el artículo es aceptado para su publicación en la RMIB, se les solicitará al autor principal o de correspondencia que revisen y firman las cartas de cesión de derechos correspondientes para llevar a cabo la autorización para la publicación del artículo. En dicho documento se autoriza a la RMIB a publicar, en cualquier medio sin limitaciones y sin ningún costo. Los autores pueden reutilizar partes del artículo en otros documentos y reproducir parte o la totalidad para su uso personal siempre que se haga referencia bibliográfica al RMIB. No obstante, todo tipo de publicación fuera de las publicaciones académicas del autor correspondiente o para otro tipo de trabajos derivados y publicados necesitaran de un permiso escrito de la RMIB.











